01、Choice of packaging materials
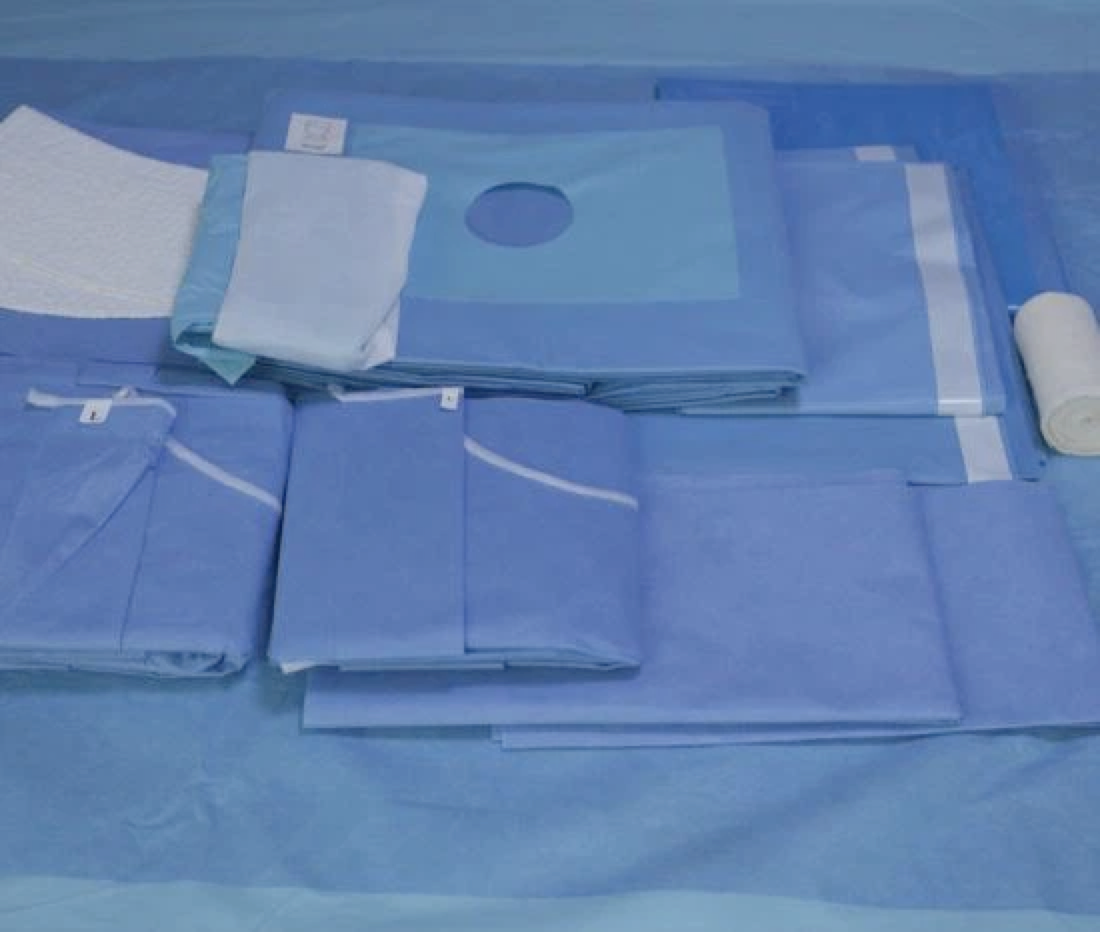
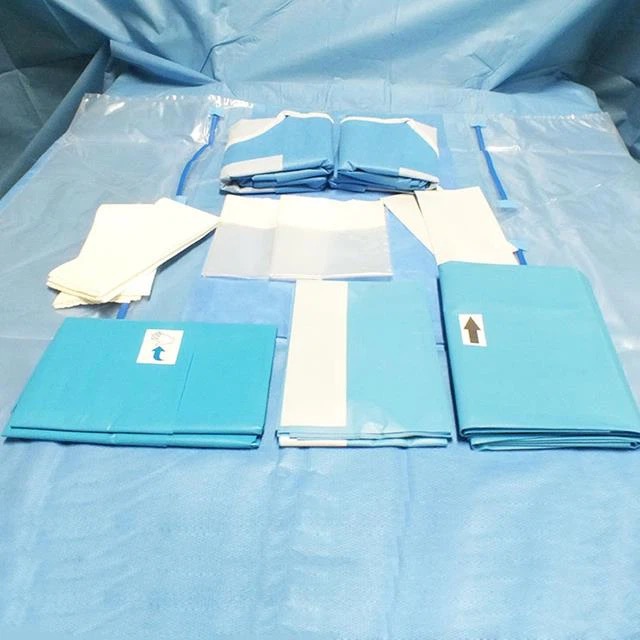
Selection of packaging materials, according to the characteristics of the device to choose the appropriate packaging materials for packaging.
For sterile articles packed with disposable medical crumpled paper and medical non-woven fabric, the expiration date should be 6 months.
For sterile articles using disposable paper-plastic packaging, the validity period should be 6 months.
02, the basic requirements of packaging
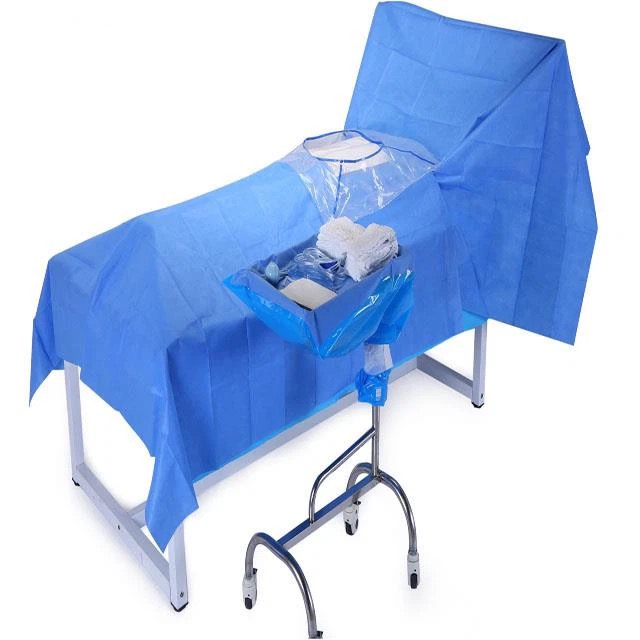
The packaging of sterilized articles is divided into closed packaging and sealed packaging.
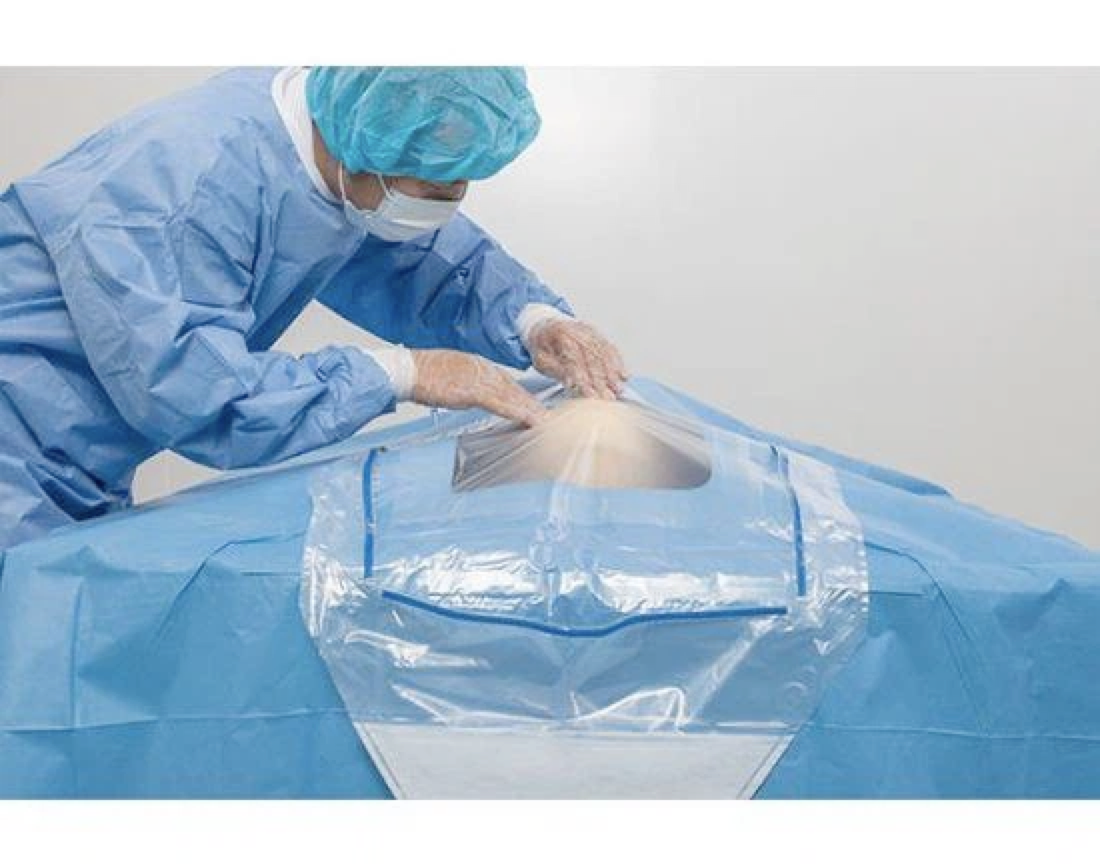
Surgical instruments are packed by closed packing method, which should be packed by 2 layers of packing materials in 2 times. Sealed packaging such as the use of paper bags, paper-plastic bags and other materials, can be used in one layer, applicable to individually packaged instruments.
If through the packaging material can be directly observed within the package sterilization chemical indicators of color change, is not placed outside the package sterilization chemical indicators, closed packaging should use special tape, tape length should be appropriate with the volume and weight of the sterilization package, elasticity is appropriate. The package should be sealed tightly to keep the closure intact.
03、Requirements for sealing package
a) There should be a sterilization chemical indicator outside the package. Highly dangerous goods sterilization package should also be placed inside the package chemical indicators; if through the packaging material can be directly observed within the package sterilization chemical indicators of color change, it is not necessary to place outside the package sterilization chemical indicators.
(b) closed package should use special tape, tape length should be appropriate with the volume and weight of the sterilization package, loose and tight. Closed package should be tight, to maintain the integrity of the closure.
(c) paper-plastic bags, paper bags and other sealed package sealing width should be ≥ 6mm, the package of instruments from the bag seal should be ≥ 2.5 cm.
(d) Medical heat sealing machine should check the accuracy of parameters and sealing integrity before daily use.
(e) Rigid containers should be set up with a safety closure device, and should be recognizable after the integrity of the sterile barrier is broken.
f) The marking of the packaging of sterilized items should indicate the name of the item, the packer and other contents. Before sterilization indicate the sterilizer number, sterilization batch, sterilization date and expiration date and other relevant information. The marking should be traceable.
04、Storage after sterilization
Sterilized objects storage racks or cabinets should be 20~25cm from the ground height, 5~10cm from the wall, 50cm from the ceiling; the use of textile materials packaged in the aseptic validity period is appropriate for 14d, the validity period is appropriate for 7d when it fails to meet the environmental standards.