Detect contamination in and microorganisms to ensure product production environment; various product quality testing instruments to ensure high product quality

Establishment of ISO13485 medical device quality system, FDA 21CFR820 rule and China sterile medical device production guidelines

With more than 3000 square meters of ISO class 8 clean room and professional generation equipment and technicians
Focusing on medical consumables
Strict standards of production
Create a better life


We have a clean room of 120,000 square meters and manufacture strictly according to ISO and CE requirements. The quality system is established in accordance with the requirements of ISO13485 and FDAQMSR (Quality Management System Regulations) as well as the on-site guidelines for sterile medical device manufacturers in China. Currently, overseas sales account for more than 95%. Healthy life, guaranteed by us.
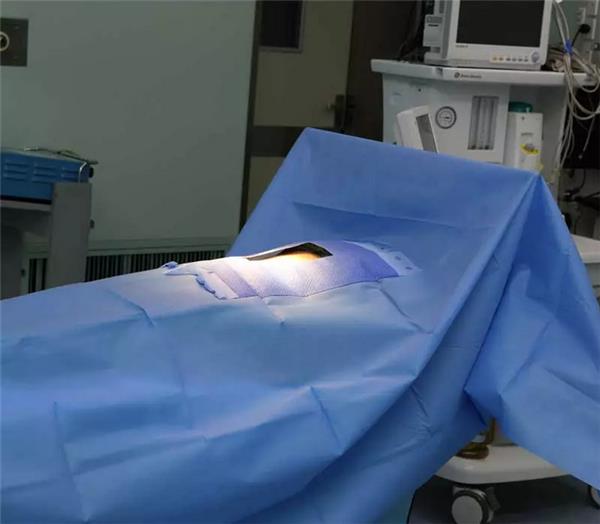
Cleaning and sterilisation of surfaces of surgical objects in the operating theatre on consecutive t
Operating theatre as a high-risk department of hospital infection, strengthen the infection control and management of operating theatre, especially clean operating theatre is an important part of the
A customer from Algeria has ordered a batch of surgical supplies, including disposable surgical bags, disposable surgical drapes, disposable surgical gowns and some surgical dressings
our team received an order from a Saudi customer, which is an order for 50,000 sets of surgical gowns, we have cooperated with surgical gloves, surgical cavity towels, surgical bags and other products
Some healthcare facilities may also use another type of LEV that captures surgical smoke close to the surgical site: connects to a dedicated centralised surgical smoke evacuation system: vents the smo