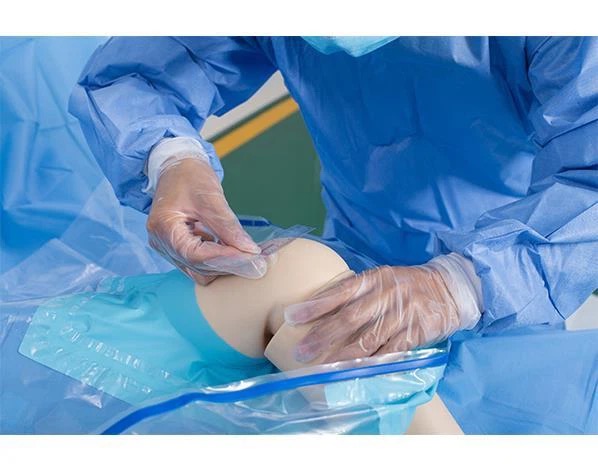


Knee Arthroscopy drape is used in the sterile knee joints. The product is non-irritating, non-toxic, odorless and has no side effects on the human body. Effectively improve surgical efficiency and reduce the risk of cross-infection.
Send FeedbackDisposable EO Sterilized Knee Arthroscopy Drape Specifications:
Product name | Knee Arthroscopy drape |
Measure | Regular size 200300cm or customized is available |
Material | SMS, Bi-SPP Lamination fabric, Tri-SPP Lamination fabric, Bi-Viscose Lamination fabric, Tri- Viscose Lamination fabric, PE film, SS |
Code | MTD-3201 Bi-Viscose Lamination fabric MTD-3202 Bi-SPP Lamination fabric MTD-3203 Tri- Viscose Lamination fabric MTD-3204 Tri-SPP Lamination fabric MTD-3205 SMS |
Label | Customized printing is available |
Feature | Disposable EO(Ethylene Oxide) |
Packaging | 1pc/sterile pouch or customized |
Brand | Medical Technology/OEM is available |
Description:
Knee Arthroscopy drape is used in the sterile knee joints procedures. The product is non-irritating, non-toxic, odorless and has no side effects on the human body. Effectively improve surgical efficiency and reduce the risk of cross-infection.
Usage: Follow the orientation markings on the arthroscopy drape, grasp the patient's leg and pull drape onto extremity to just above the knee, next unfold the drape. Form the pouch move the lower panel of the pouch to below the knee. The drape provides a large fluid collection pouch, it includes a port used to connect suction and tabs for holding cords.
Features:
1) High ventilation and waterproof.
2) Disposable material that convenient and comfortable, protected from crossing infection, prevent the operation infection effectively.
3) Packaging by imported medical paper to ensured the drape can restored safety until the valid date.
4) Strictly follow the operation of the scientific method of fold.
Product detail:
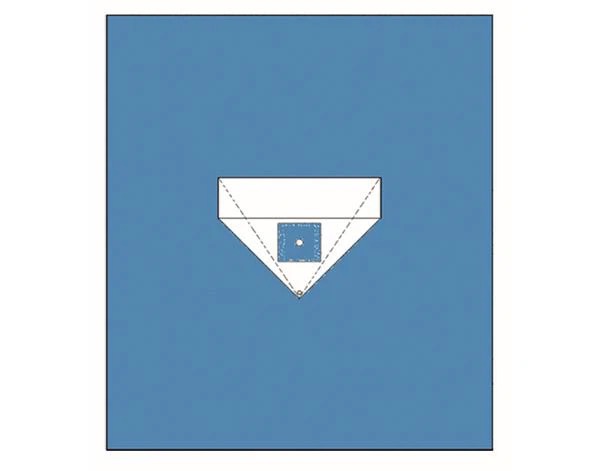
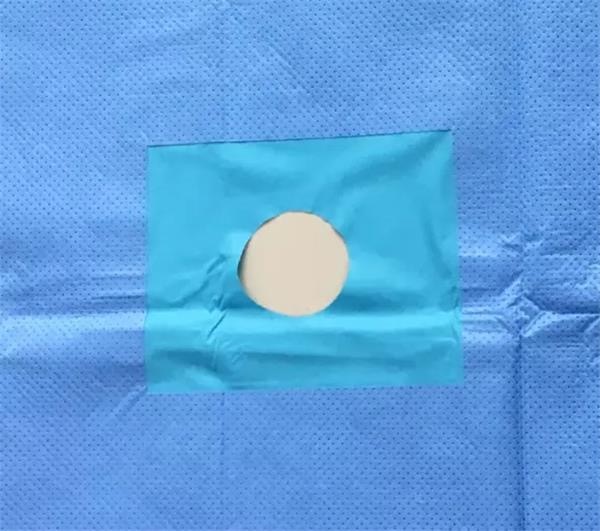
FAQ
Q1: Do you have CE Certificate ?
A1: Yes, we have. We have ISO 13485 and CE Certificate.
Q2: What is the expired date ?
A2: The validity is five years, and the case should be stored in a dry.
Q3: How can I get samples to test your quality ?
A3: Please offer us the exactly specific details, we will send samples to you for your options.
Q4: Can you make OEM or ODM service ?
A4: Yes, we can, please send us your design or your request, we can customize the gown according to your design.
Our Factory 
Our Strengths
R&D: Development and production of high quality and high value medical dressings and nonwoven products
Our factory: the production base covers an area of more than 50,000 square meters, with ISO8 clean plant and more than 4,000 meters of ethylene oxide sterilization center
Our equipment: focus on scientific and technological innovation, the introduction of international advanced production equipment and technology
Our qualification: Strict quality control, obtained ISO13485 and CE certificate.
Our service
1. Accept orders no matter how big or small they are.
2. Provide FOB, CFR, CIF, EXW terms.
3. Accept T/T, L/C, D/P.
4. Provide free samples.
5. Provide OEM customized service with more choices of materials, patterns, sizes and packaging to satisfy your personalized needs.
6. Our factory, perfect management system, high quality and favorable price.
7. Fast delivery, standardized and efficient production.
8. Good after-sales service, you have any problems quickly dealt with.