EN 13795 closes the missing technical link to compliance with the regulations of the European Council Directive 93/42/EEC that is often referred to as Medical Device Directive (MDD). The Medical Device Directive was published in 1993 and became mandatory in all European and EFTA countries in 1998 superseding prior existing national legislation. MDD applies to all medical devices and describes the essential requirements in a broad manner.
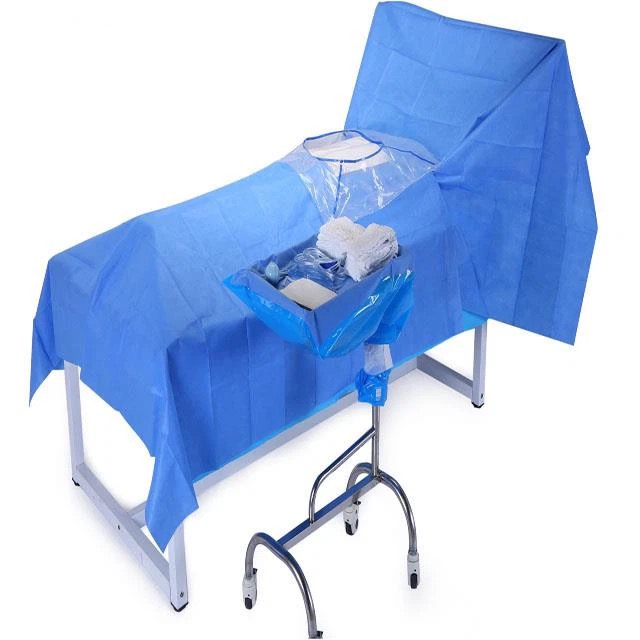
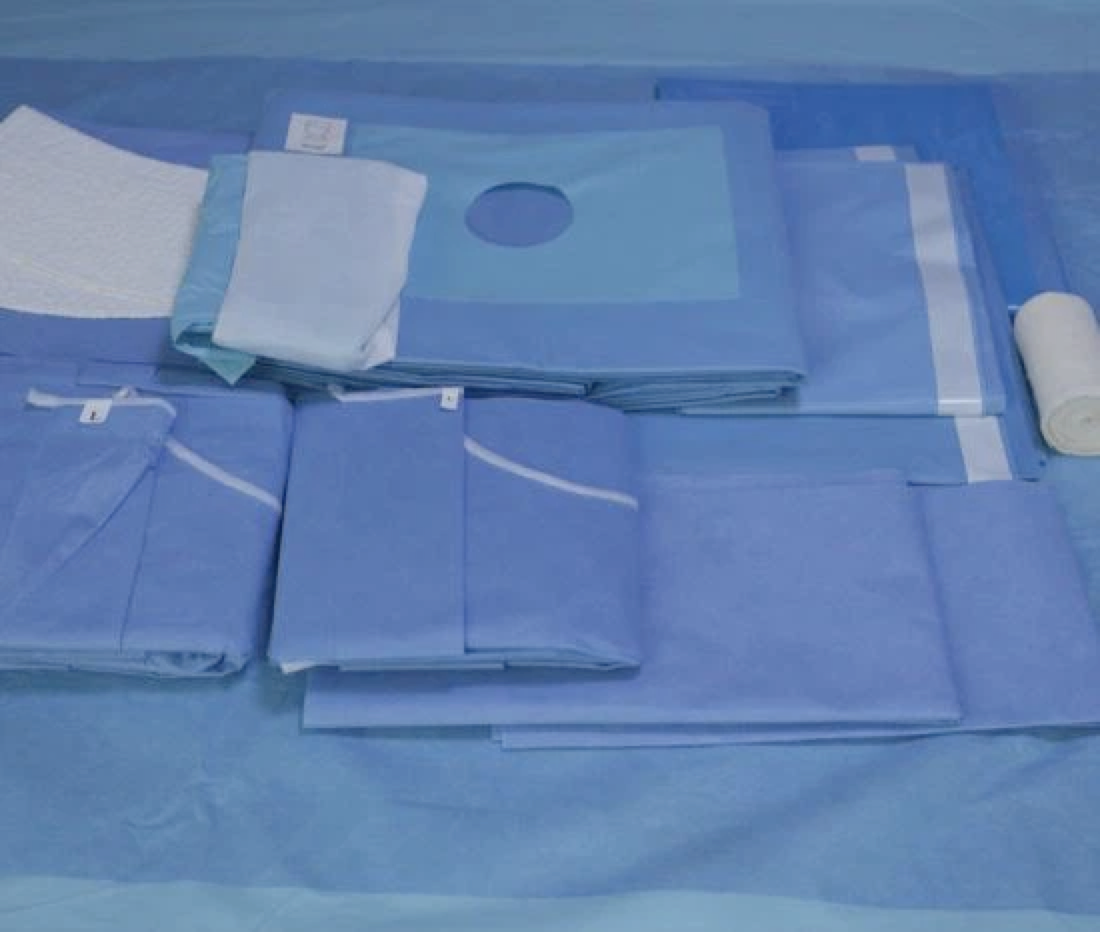
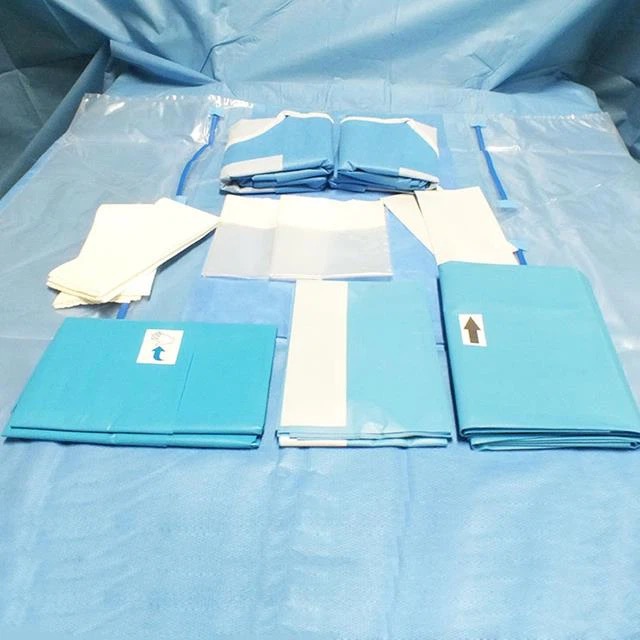
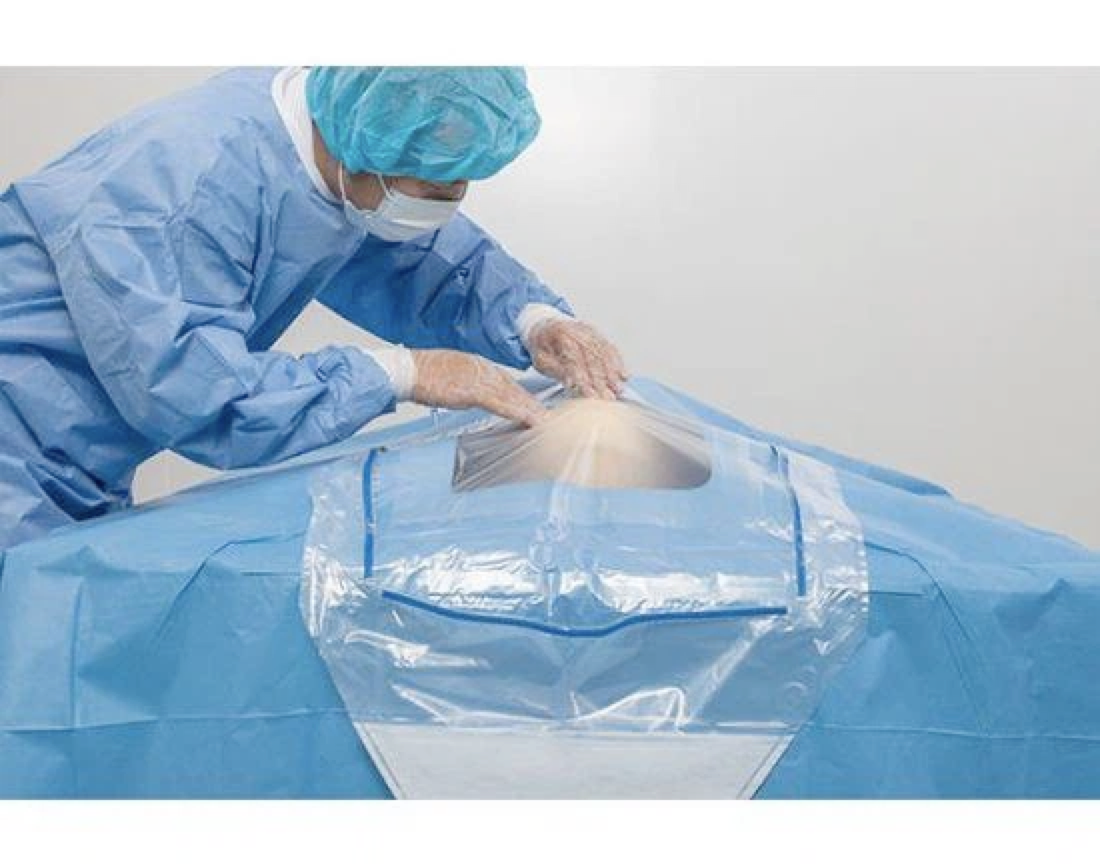
This Standard specifies information to be supplied to users and third party verifiers addition to the usual labelling of Medical Devices concerning manufacturing and processing requirements. It gives general guidance on the characteristics of single-u and re-usable surgical gowns, surgical drapes and clean air suits used as medical devices for patients, clinical staff and equipment. It is intended to prevent the transmission of infectious agents between patients and clinical staff during surgical and other invasive procedures.
The essential requirements regulate the design and construction of medical devices and clarify key parameters for ensuring that medical devices will not compromise the safety and health of patients and healthcare professionals. These parameters include the conditions for their safe use, storage, transport and labelling as well as for their chemical, microbiological and physical properties. Each device is classified as Class I, Class II or Class I depending on its intended purpose and its associated risks. Class I covers non-invasive devices such as surgical gowns, drapes and clean air suits. Classes II and III deal with devices that bear a higher risk potential for patients and healthcare workers.
The new Standard defines the essential requirements for surgical gowns, drapes and clean air suits. The European Standard EN 13795 consists of 3 separate parts.
PART ONE GENERAL REQUIREMENTS FOR MANUFACTURERS, PROCESSORS AND PRODUCTS EN 13795-1
PART TWO TEST METHODS EN 13795-2
PART THREE PERFORMANCE REQUIREMENT AND PERFORMANCE LEVEL EN 13795-3
Contact us: sales@hnmedtech.com